Pharmacokinetic and Toxicokinetic Studies
Pharmacokinetic (PK) studies describe the time course of drug concentration in the body following in vivo administration. Key determinants of a drug's PK include absorption, distribution, metabolism, and elimination (ADME). As a variation of PK, toxicokinetic (TK) studies assess the relationship between the toxic dose in vivo and its toxic effects. A PK/TK study involves dosing animals and collecting blood or other biological samples at predefined time points, based on which a drug concentration-time profile and relevant PK/TK parameters are generated.
Creative Bioarray provides professional PK/TK testing services to help our customers choose pharmaceutical compounds and effective and safe dosing regimens.
Animal Species
- Rodents
Mice, Rats, Guinea pigs
- Non-rodents
Dogs, Minipigs, Non-human primates
Study Design
The study is typically divided into different groups based on the selected dosing method. A standard study investigating the PK/TK of a drug compound in rats is as follows.
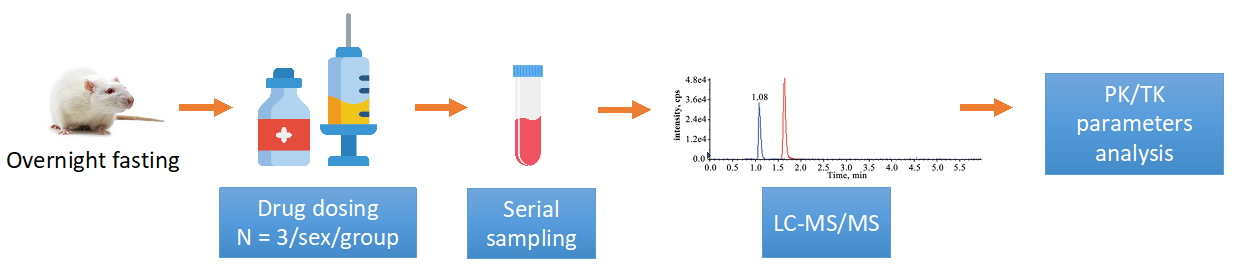 Figure 1. Standard study investigation of drug PK/TK
Figure 1. Standard study investigation of drug PK/TK
According to different research purposes, our experimental design can be adjusted in the following aspects, such as
- Additional animals
- Additional or custom time points
- Crossover studies
- Dose proportionality studies
- Multiple-dose studies
- Fed/food effects
- Special populations
- Gender effects
- In-life observations
Drug dosing routes
- Default: intravenous (iv) and oral administration (po)
- Others: intraperitoneal (ip), intramuscular (im), and subcutaneous (sc) injections, iv cannulation
Dosing Methods
While a single dose at the provided dosage is usually given to each animal, other drug dosing methods can be included to accommodate your specific needs.
- Additional dosage groups
- Vehicle dosing
- Repeated dosing
- Cassette dosing for rapid evaluation of multiple compounds
Sample Collection
- Serial or terminal sampling
- Blood plasma/serum
- Microsampling
- Tissues or biological fluids sampling
Endpoints
LC-MS/MS measures the concentrations of the test compound in plasma to generate a concentration-time profile for each dosing group, based on which PK/TK parameters (including Cmax, Tmax, AUC0-t, AUC∞, absolute BA, Ke, t1/2, C0, Vd, and CL, etc.) are calculated using non-compartmental analysis. One- or Two-compartment analyses may be applied when appropriate.
With deep PK/TK testing experience, Creative Bioarray helps you choose the best industry-standard instruments and software that are most suitable for your project, whether it is assay transfer, development, verification, sample analysis, or combination.
Quotation and ordering
If you have any special needs or questions regarding our services, please feel free to contact us. We look forward to cooperating with you in the future.
References
- Schrag, M.; Regal, K. Chapter 4—Pharmacokinetics and Toxicokinetics. In A. S. Faqi (Ed.), A Comprehensive Guide to Toxicology in Nonclinical Drug Development (Second Edition) (pp. 69-106). Academic Press. (2013).
- Tietje, C.; Brouder, A. (Eds.). International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In Handbook of Transnational Economic Governance Regimes (pp. 1041-1053). Brill | Nijhoff. (2010).

