Hepatocyte Stability Assay
With a highly automated approach, Creative Bioarray provides highly cost-efficient hepatocyte stability assay as one of our in vitro metabolism services and delivers consistent and high-quality data.
Hepatocyte Stability Assay Introduction
- Drug detoxification and drug metabolism are primarily carried out in the liver. About three-quarters of the drugs cleared via metabolism are metabolized and cleared by hepatic cytochrome P450 (CYP)-mediated metabolism (Wienkers, 2005).
- Hepatocytes contain the full range of both phase I and phase II hepatic drug-metabolizing enzymes thus can serve as a perfect model to determine the in vitro clearance of a compound.
- Primary human hepatocytes are considered the Gold Standard for metabolism studies since they contain all the hepatic enzymes, transporters, and co-factors needed for drug metabolism.
- Hepatocytes can be used in metabolic stability assays, drug efflux, uptake studies, metabolite identification, CYP induction/inhibition studies, etc.
Brief Protocol
- Hepatocytes are incubated with the test article at 37 °C for different time periods.
- Samples are removed at each indicated time point during incubation, and the reaction is terminated with a stop solution.
- The samples are then centrifuged, and supernatants are collected for LC-MS/MS analysis. The clearance of the test article is monitored over a time period of 60 minutes (or longer).
- Drug clearance rate and half life are then calculated using the obtained data.
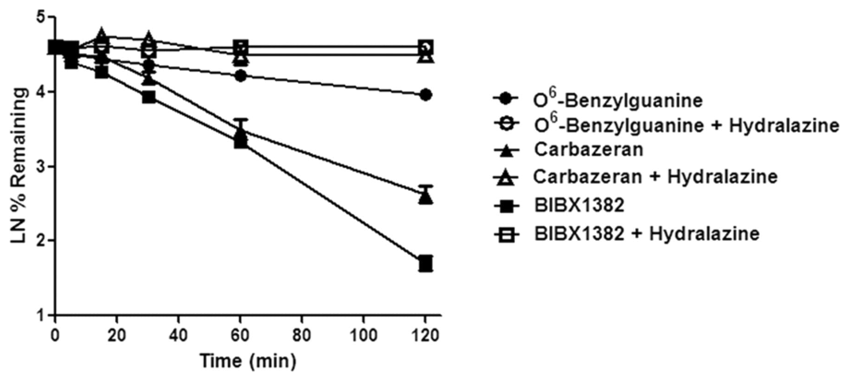 Figure 1. Hepatocyte stability profile of three compounds (Hutzler, 2012).
Figure 1. Hepatocyte stability profile of three compounds (Hutzler, 2012).
Species
- Rodents
- Mouse
- Rat
- Guinea pig
- Non-rodents
- Human
- Rabbit
- Dog
- Minipig
- Other species according to customer requirements
Case Study
- Thawing of cryopreserved human hepatocytes
The hepatocyte vial was removed from the liquid nitrogen tank and immersed into the water bath. When only a small ice crystal remained, the cell suspension was transferred to the tube with the preheated suspension medium. The cells were centrifuged at 42 g for 5 min. After centrifugation, the cells were resuspended gently. The cells were counted, and viability was determined using trypan blue staining.
- Methods and Results
Compounds are incubated in the presence of hepatocytes at 37°C. The reactions are performed in two replicates per compound and terminated with acetonitrile at each of the 6 sampling time points: 0, 5, 15, 30, 45 min incubation. The samples are then centrifuged, and the relative parent compound depletion is determined by LC-MS/MS analysis.
| Test Article | CLint (µL/min/106 Cells) | Clearance Category |
| ketanserin | 33.1 | High |
| Imipramine | 4.7 | Moderate |
| Sample#1 | 38.3 | High |
| ||
Quotation and Ordering
Creative Bioarray's hepatocyte stability assay can be further extended to metabolite profiling and give out more information about the metabolism of your compound. Liver microsome stability assay and S9 stability assay are also available at Creative Bioarray. To find out more about our services, please feel free to leave a message below or contact us. Our well-experienced experts will be more than happy to help.
References
- Wienkers, Larry C., and Timothy G. Heath. "Predicting in vivo drug interactions from in vitro drug discovery data." Nature reviews Drug discovery 4.10 (2005): 825-833.
- Hutzler, J. Matthew, et al. "Characterization of aldehyde oxidase enzyme activity in cryopreserved human hepatocytes." Drug Metabolism and Disposition 40.2 (2012): 267-275.

