Generation of NRF2 Knockout iPSC Clones Using CRISPR/Cas9 Editing
Stem Cell Research. 2023 Jun; 69: 103090.
Authors: Merkert S, Haase A, Dahlmann J, Göhring G, Waqas FH, Pessler F, Martin U, Olmer R.
INTRODUCTION
The nuclear factor erythroid 2-related factor 2 (NFE2L2, known as NRF2) regulates the expression of antioxidative and anti-inflammatory proteins. To investigate its impact during viral infections and testing of antiviral compounds, we applied CRISPR/Cas9 editing to eliminate NRF2 in the human iPS cell line MHHi001-A and generated two NRF2 knockout iPSC clones MHHi001-A-6 and MHHi001-A-7.
METHODS
- Cell culture. The iPS cells were cultured on plates at 37°C in a 5% CO2 incubator. Single-cell monolayer cultures were split with a seeding density of 3.6x104 cells/cm2, and colonies were passaged as clumps at a ratio of 1:6. Cell culture supernatants were tested for mycoplasma contamination.
- CRISPR/Cas9 mediated knockout and clone generation. Suitable crRNAs for the NRF2 target sequences were designed and selected based on their low probability for off-target effects. Both crRNAs, the trancrRNA (10 µM each), and 5 µg Cas9 protein were mixed into a 10.5 µl RNP complex. For transfection, 6x105 iPSCs were resuspended in 100 µl buffer, mixed with the preformed RNP complex, and electroporated with one pulse at 1700 V for 20 ms with the transfection system. After 3 days, limiting dilution was performed on coated 96-well plates to generate single-cell clones. After 8-10 days, resulting clones were transferred to a 48-well plate. PCR analysis was performed to screen for positive clones.
- Genotyping and sequencing. To determine the knockout sequence, the targeted region of the NRF2 gene was amplified and the 410 bp PCR products were sent for Sanger sequencing.
- Immunofluorescence staining and flow cytometry. For immunocytochemistry, cells were fixed in 4% PFA for 20 min at room temperature (RT) and permeabilized with a blocking solution (20 min, RT). The cells were incubated with primary antibodies for 1 h, with secondary antibodies for 30 min, and stained with DAPI for 15 min (all at RT). For flow cytometry, cells were either stained directly with surface antibodies for 15 min at 4°C or fixed with 4% PFA for 10 min. Fixed cells were permeabilized with ice-cold 90% methanol for 20 min and stained with antibodies overnight at 4°C.
- Karyotyping. Karyotype analysis was performed for both clones at passage 11 after targeting. 20 metaphases were analyzed using fluorescence R-banding at a minimum of 300 bands.
- STR analysis. Tested loci include D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, AMEL, vWA, D8S1179, TPOX, FGA.
- In vitrodifferentiation. IPSCs were harvested from mouse embryonic fibroblasts by Collagenase IV and seeded into ultra-low attachment plates in IMDM supplemented with 20% FSC, 1% NEAA, 1 mM L-Glutamine, and 0.1 mM β-mercaptoethanol. After 7 days EBs were transferred onto gelatin-coated 12 well plates for another 14 days before PFA fixation and immunostaining. To check for the absence of NRF2 protein, single-cell monolayer iPSC cultures were differentiated into ECs as previously described.
- Browse our recommendations
Creative Bioarray provides professional products and services, including but not limited to the following.
| Product/Service Types | Description |
| iPSC Generation | Creative Bioarray employs advanced iPSC reprogramming factor delivery by episomal vectors, viral vectors, and other iPSC reprogramming methods (mRNA, protein, and chemical reprogramming) to help you obtain the desired iPSC. |
| Immunohistochemistry (IHC), Immunofluorescence (IF) Service | By providing high-quality immunohistochemistry (IHC) and immunofluorescence (IF) services to customers for many years, Creative Bioarray will give you comprehensive service in regular and customized IHC and IF services. |
| Cell Line Authentication | Creative Bioarray STR profiling is critical for verifying the identity of human cell lines, ensuring the uniqueness of the cell line, and detecting laboratory errors such as misidentification and cross-contamination of lines. |
RESULTS
- To further investigate the cytoprotective NFR2 pathway in viral infections like SARS-CoV-2 and to assess the efficacy of NRF2-activating compounds we generated NRF2 knockout clones from the human iPSC line MHHi001-A.
- Therefore, two suitable guide RNAs were designed for the integration of double-strand breaks in exon 2 and exon 5, resulting in the deletion of 2276 bp within the NRF2 gene. Sanger sequencing revealed the deletion of 2276 bp in both clones, resulting in an open reading frame shift that causes amino acid changes and a premature stop codon.
- The clones exhibited normal human karyotype 46, XX, and classical human pluripotent stem cell morphology. They highly expressed pluripotency markers like OCT4, NANOG, SSEA-4, and TRA-1-60, at passage 10 after targeting, as demonstrated by immunocytochemistry and flow cytometry. Both clones were capable of differentiation into all three germ layers. The differentiated cells showed expression of endoderm (AFP, SOX17), mesoderm (TBXT, ACTA2), and ectoderm markers (PAX6, TUBB3).
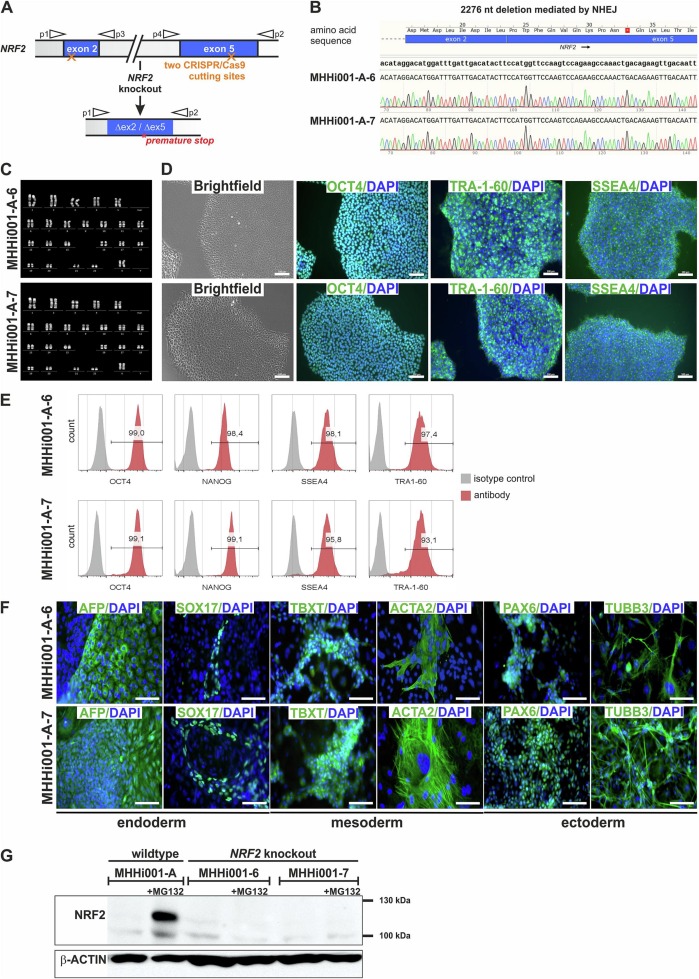 Fig. 1 Generation and characterization of two NRF2 knockout iPSC lines.
Fig. 1 Generation and characterization of two NRF2 knockout iPSC lines.
SUMMARY
Since activation of the NRF2 cytoprotective and immunomodulatory signaling pathway is not yet completely understood, we generated NRF2 knockout iPS cell clones. After differentiation into lung epithelia and vascular endothelial cells, these lines will help to investigate the stress response after viral infection with influenza viruses, corona-viruses, and other viral pathogens, and to develop and test new antiviral compounds.
RELATED PRODUCTS & SERVICES
Reference
- Merkert S, et al. (2023). "Generation of two human NRF2 knockout iPSC clones using CRISPR/Cas9 editing." Stem Cell Res. 69, 103090.