Senescence Associated β-galactosidase Assay
Normal mammalian cells divide in a fixed number of population doublings and eventually enter an arrested state in which the cells are still alive, but do not proliferate in response to mitogens, and exhibit a characteristic expanded, flattened morphology.
Senescence associated β-galactosidase (SA-β-gal) is a widely used biochemical marker for assessing cellular senescence. SA-β-gal can be detected by 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) at pH 6.0, which inhibit lysosomal β-galactosidase activity sufficiently to ensure that non-senescent cells remain unstained.
This method to detect SA-β-gal is a convenient, single cell-based assay that can recognize senescent cells even in heterogeneous cell populations and aging tissues such as skin biopsies from older individuals.
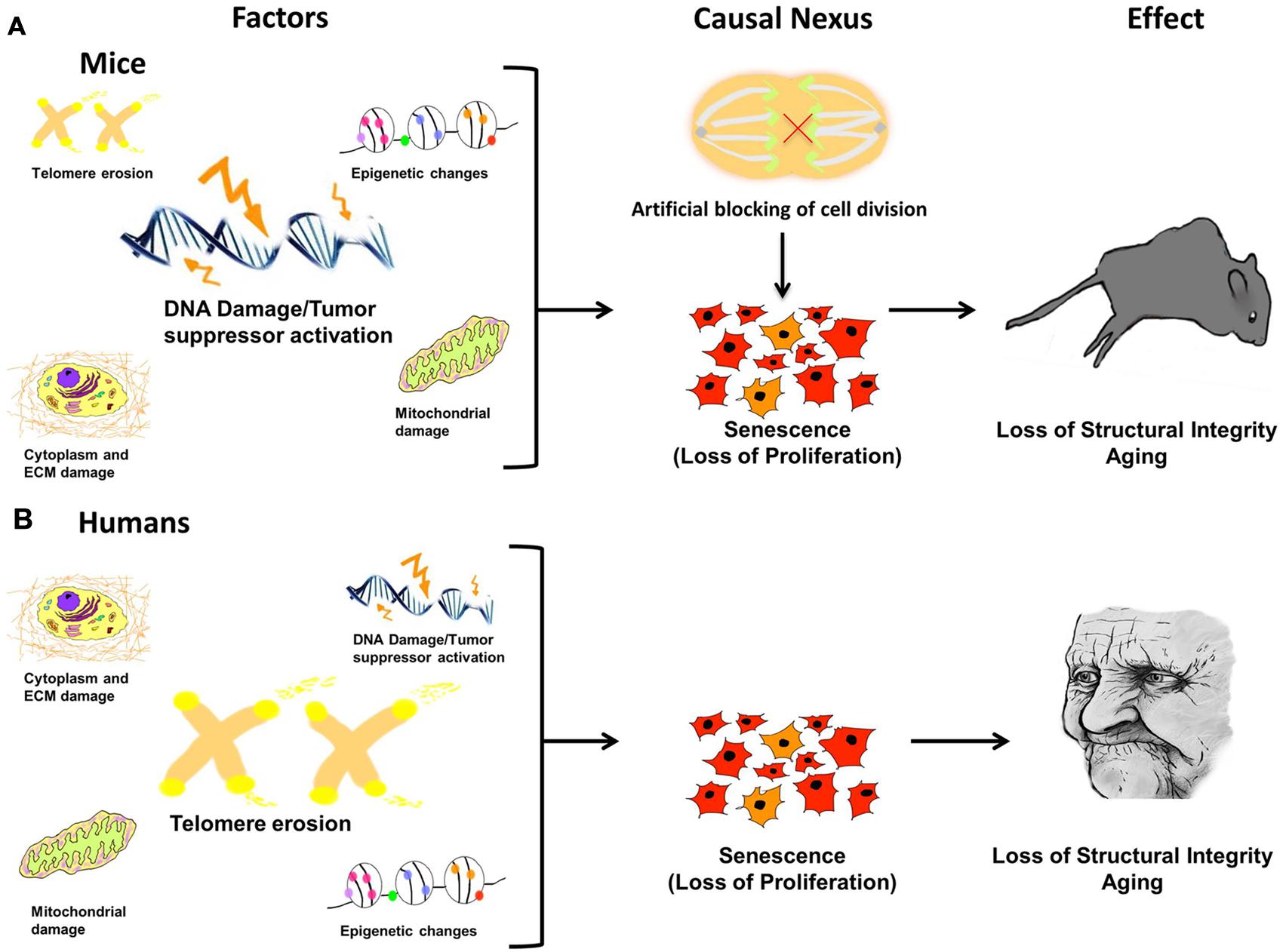 Figure 1. Damage leads to causal nexus leads to effects.
Figure 1. Damage leads to causal nexus leads to effects.
Materials
Phosphate buffered saline (PBS)
Paraformaldehyde (PFA)
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal)
Potassium ferrocyanide
Potassium ferricyanide
Sodium hydroxide
Dimethylformamide (DMF)
Sodium chloride
Magnesium chloride
Dibasic sodium phosphate
Citric acid
Sodium phosphate
Reagent Preparation
4% paraformaldehyde (PFA)
- Dissolve 4 g PFA in 100 mL of PBS with continuous stirring and add 20 μL 1 M sodium hydroxide to dissolve the residual PFA.
- Aliquot and freeze at -20°C for long-term storage.
Senescence associated β-galactosidase (SA-β-gal) staining solution
- Firstly, prepare the following stock solutions.
1) To prepare 10% X-gal by dissolving 1 g X-gal in 10 mL DMF. Store the stock X-gal solution at -20°C.
2) For 400 mM citric acid/sodium phosphate solution, adding 36.85 mL 0.1 M citric acid to 63.15 mL 0.2 M dibasic sodium phosphate. Verify the pH and adjust to pH 6.0 with 0.1 M citric acid, if necessary.
3) Potassium ferricyanide and potassium ferrocyanide are dissolved in water at 0.5 M and store the stock solutions in the dark at 4°C. - Secondly, prepare the SA-β-gal staining solutions.
Making appropriate dilutions of stock solutions with water to give a SA-β-gal staining solution containing 0.1% X-gal, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM sodium chloride, and 2 mM magnesium chloride in 40 mM citric acid/sodium phosphate solution, pH 6.0.
Assay Procedure
- The SA-β-gal staining assay may be performed in a 24-well plate.
- Prepare each sample in triplicate.
- After the cell manipulation, aspirate the cell culture medium and wash the cells twice with PBS (500 μL per well).
- After the last rinse, replace the PBS with 250 μL of 4% PFA for fixation.
- Incubate the cells for 5 min at room temperature.
- Aspirate the 4% PFA and wash the cells twice for 5 min each with gentle shaking in 500 μL PBS.
- Add 250 μL SA-β-gal staining solution to each well.
- Incubate the cells in the dark in a 37°C incubator (not in a CO2 incubator).
- Terminate the reactions when the cells are stained blue-green, as visualized under a bright-field microscope.
- Aspirate the staining solution and replace with distilled water to terminate the reaction.
- Wash the cells twice in distilled water.
- After the last wash, adding 500 μL of distilled water to each well and observe the plate under an inverted bright-field microscope.
- Capture images of cells in each well.
- Images may be printed for counting the total cell number or count the stained cells on a computer monitor, which may give better distinction between the unstained and stained cells.
- Represent the SA-β-gal positive cells as a percentage of the total cell number.
Note: A trial may need to be carried out to determine the optimal time of incubation with SA-β-gal staining solution for each of the cell lines.
Frequently Asked Questions
Q1: Can frozen tissue sections be used with this method?
A1: This method has been used for skin sections successfully. Briefly, the tissue was frozen in liquid nitrogen, and mounted in OCT. The thin sections (4 µm) were cut, mounted onto glass slides, fixed in 1% formalin for 1 min at room temp, washed in PBS, immersed overnight in SA-β-gal staining solution. Then you can view under bright field.
Q2: Can paraffin tissue sections be used with this method?
A2: This has not been determined yet.
Q3: Which tissues have been tested?
A3: Skin tissue section (frozen).
Q4: Does this method detect transient expression of p53 (3-5 days) or longer term expression?
A4: The method will detect senescent cells. If the p53 expressing cells become senescent, then the method should detect. It does not matter what causes senescence, but as long as cells become senescent, this method will work.
Q5: Why are some crystals formed after leaving overnight?
A5: These crystals are salt crystals formed due to the solvent evaporation. Our recommendation is to keep the plate sealed when is left overnight.
References
- Koji Itahana, et al.; Methods to detect biomarkers of cellular senescence: The senescence-associated beta-galactosidase assay. Methods in Molecular Biology, 2007, 371: 21-31.
- Debacq-Chainiaux F. et al.; Protocols to detect senescence-associated beta-galactosidase (SA-β-gal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc, 2009, 4(12): 1798-1806.
- Bhatia-Dey D. et al.; Cellular senescence as the causal nexus of aging. Front Genet, 2016, 7: 13.
Cell Services:
Cell Line Testing and Assays:

